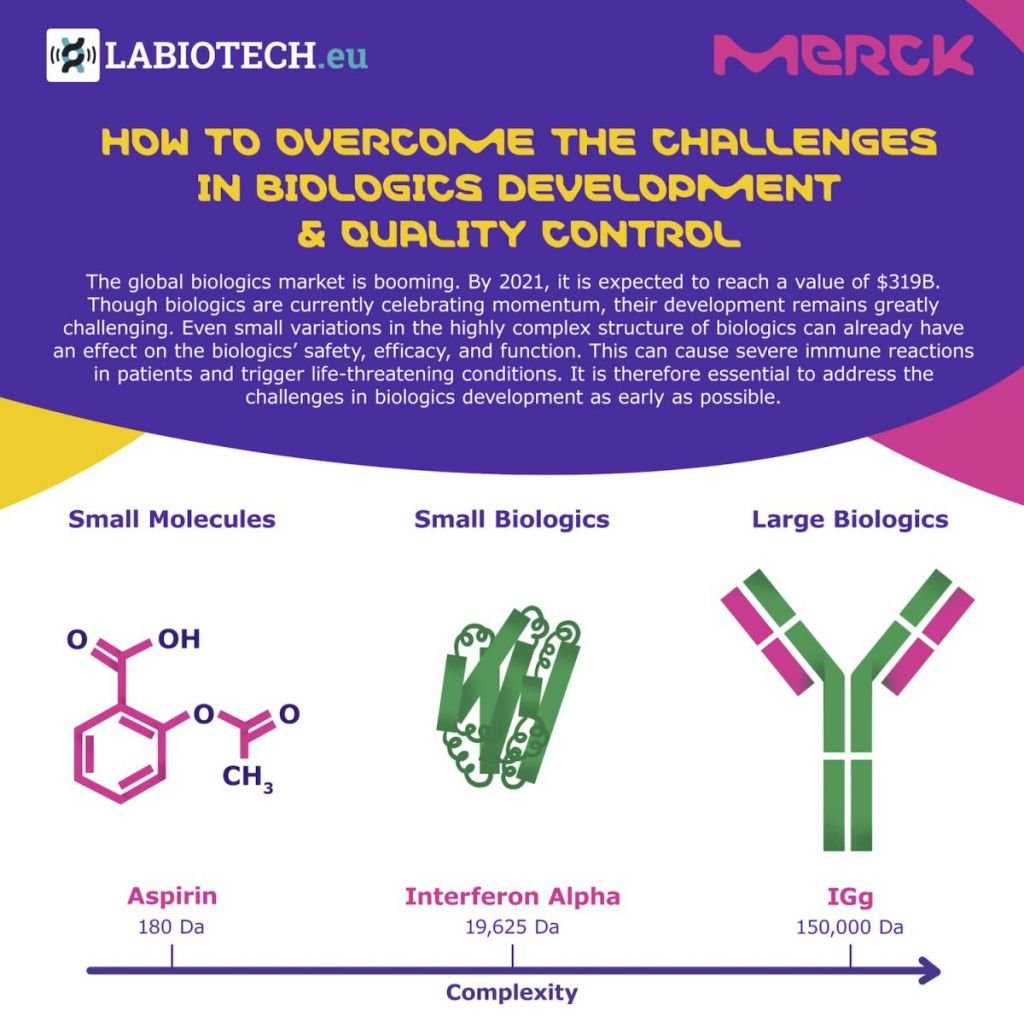
By 2021, the global biologics market is expected to reach a value of $319B. But although they are currently celebrating a momentum, biologics development and quality control remain greatly challenging. To prevent severe immune reactions in patients that could trigger life-threatening conditions, these challenges need to be addressed.
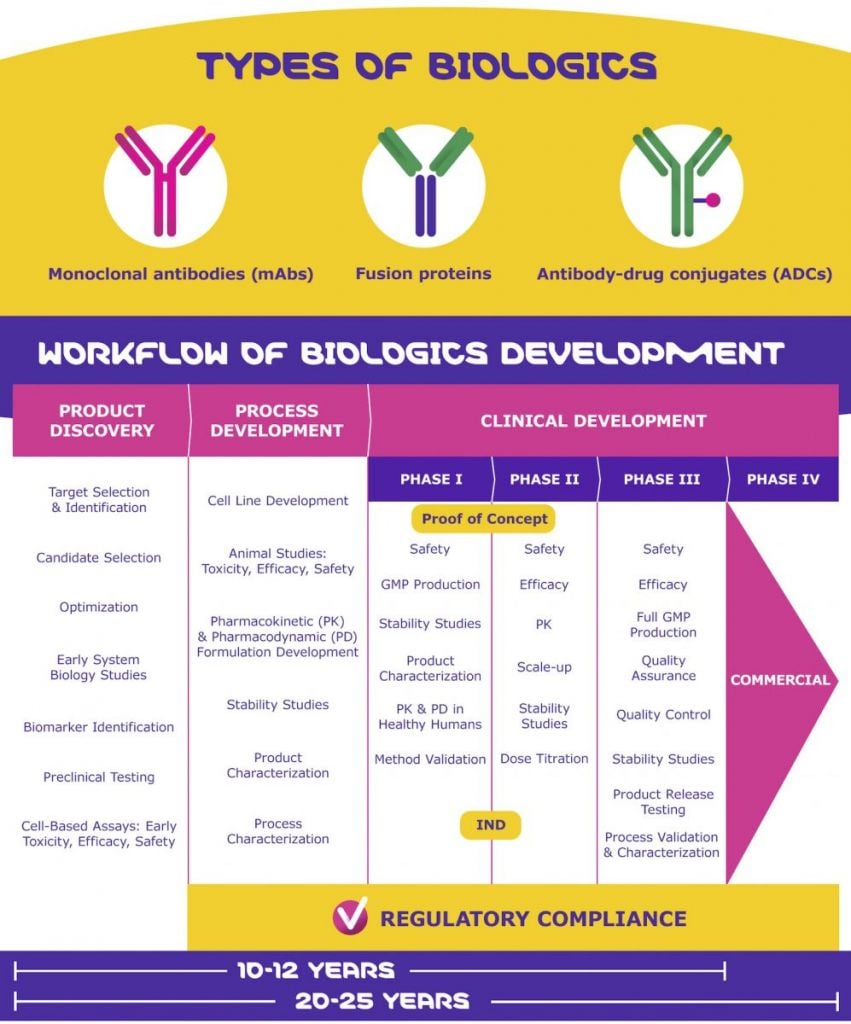
Because of their great molecular complexity, the smallest variations in a biologic’s structure can greatly affect its safety, efficacy, and function. Consequently, the development process of biologics and their quality control throughout product discovery, process development, and clinical development is highly complex.
The whole biologics development process from product discovery through commercialization can take up to 12 years. Why? Because not only is the development process highly complex, but it also comes with a number of challenges.
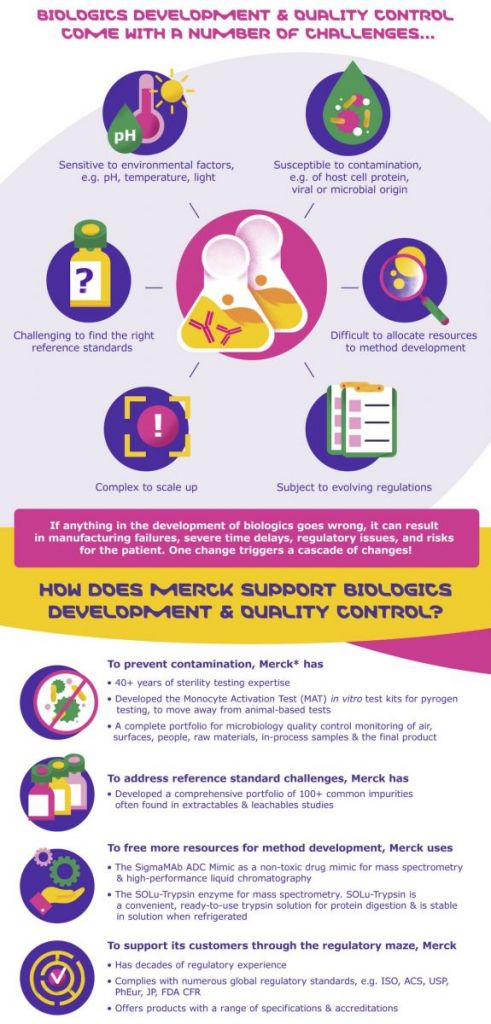
For example, biologics are sensitive to environmental factors, susceptible to contamination, difficult to scale up, and subject to constantly evolving regulations. So with every step of the way, biologics need to be carefully protected and controlled.
Together with Merck*, we created this wonderfully colorful infographic to show you how biologics development and quality control can be mastered with state-of-the-art technologies and decades of experience.
You can download a free version of the infographic here!
Each of Merck‘s life science portfolio brands focuses on a different segment of biologics development and quality control. Although each brand has its own unique way of doing things, together, they have a common goal: to solve the toughest challenges in the life sciences.
Merck’s brands include Millipore®, Supelco®, Milli-Q®, Sigma-Aldrich®, and BioReliance®. With decades of experience, they can master the challenges of biologics development and quality control by preventing contamination, addressing challenges of reference standards, freeing resources for method development, and supporting its customers with regulatory advice.
Tackle biologics development and quality control now! Benefit from Merck’s expertise and check out its latest educational content and products here!
*The life science business of Merck operates as MilliporeSigma in the U.S. and Canada
Author: Larissa Warneck, Science Journalist at Labiotech.eu
Design: Elena Resko