Diabetes has become an epidemic, sentencing over 460 million people worldwide to lifelong medication. Science is striving to find a diabetes treatment that can cure this chronic disease, but how close are we?
Diabetes is the major cause of blindness, kidney failure, heart attack, and stroke. It is estimated that the number of people affected by diabetes will rise to 700 million by 2045. This has led the World Health Organization to consider diabetes an epidemic.
Despite its huge impact on the global population, there is still no cure for any type of diabetes. Most treatments help patients manage the symptoms to a certain extent, but diabetics still face multiple long-term health complications.
Diabetes affects the regulation of insulin, a hormone required for glucose uptake in cells, resulting in high levels of blood sugar. While there are some similarities in symptoms, the two main types of diabetes develop in different ways.
Type 1 diabetes is an autoimmune disease that destroys insulin-producing beta-pancreatic cells. In contrast, patients with type 2 diabetes develop insulin resistance, meaning that insulin is less and less effective at reducing blood sugar.
The biotech industry is striving to develop new diabetes treatments and chasing the holy grail: a cure. Let’s have a look at what’s brewing in the field and how it will change the way diabetes is treated.
Type 1 diabetes
Replacing missing cells with cell therapy
Although still in the very early stages of development, cell therapy is one of the biggest hopes towards developing a cure for diabetes, especially for type 1 diabetes. Replacing the missing insulin-producing cells could potentially recover normal insulin production and cure patients.
However, early attempts to transplant pancreatic cells have largely failed, mostly due to immune reactions that reject and destroy the implanted cells. The lack of donors is also a limitation.
One of the most advanced alternatives comes from the Diabetes Research Institute in the US, which is developing a bioengineered mini-organ where insulin-producing cells are encapsulated within a protective barrier. This mini-pancreas is then implanted into the omentum, a part of the abdominal lining. A phase I/II trial is ongoing, but the DRI announced its first successful results in 2016, revealing that the first patient in Europe treated with this approach no longer requires insulin therapy.
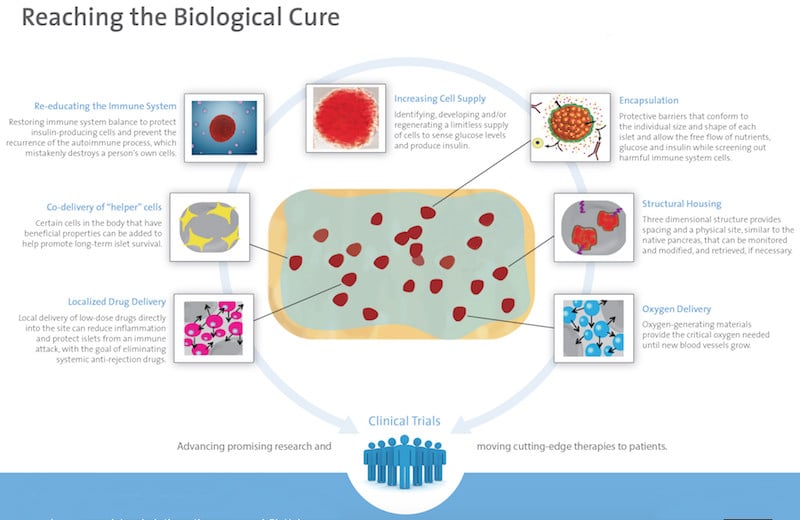
“This can be the beginning of a new era in islet transplantation. Our ultimate goal is to prevent the need for lifelong anti-rejection therapy,” stated Camillo Ricordi, Director of the DRI.
A similar device is being developed by the US company ViaCyte, in collaboration with the nonprofit organization JDRF. A phase I/II trial is ongoing and has already proved the device is safe. The company is now working on improving the engraftment of insulin-producing cells.
Together with the Swiss gene-editing company CRISPR Therapeutics, ViaCyte is also due to launch a phase I trial of an off-the-shelf beta cell replacement therapy, where beta cells are reintroduced into diabetes patients to improve insulin production.
A similar approach is being pursued by German firm Evotec, which has a stem cell-based beta cell replacement therapy in preclinical development. Evotec was originally co-developing the therapy with Sanofi but, since Sanofi withdrew from the collaboration last year, Evotec will carry on the development alone.
Overall, big pharma are still in the earlier stages of developing their own cell therapy approaches for diabetes. For example, Novo Nordisk, one of the largest providers of diabetes treatments, is bidding for stem cells and an encapsulation device, with the first in-human clinical trial currently in planning.
Although the promises are big, these technologies are still far from the market. First, clinical trials have to show they work. In addition, the price could be steep, as cell therapies for other applications, such as oncology, come with six-figure price tags and are finding it difficult to get reimbursement from health insurance companies. Considering that compared to cancer, diabetes is, for the most part, not an immediately life-threatening disease, health insurers in some countries might be reluctant to cover the treatment.
Attacking the origin with immunotherapy
In type 1 diabetes, insulin-producing cells are progressively destroyed by the immune system. Stopping this process early enough could preserve the cells and provide a cure.
That is the goal of Imcyse, a Belgian company running a phase I/II clinical trial with an immunotherapy designed to stop type 1 diabetes by specifically killing the immune cells that destroy the pancreas. A phase I trial showed there were no major safety issues related to the immunotherapy and some clinical benefits were also revealed.
“Early after diagnosis, between three to six months, it is estimated that around 10% of the insulin-producing cells are still alive and producing insulin. After stopping the autoimmune process, the remaining beta cells would be protected and could continue producing insulin,” explained Pierre Vandepapelière, CEO of Imcyse.
Belgian company ActoBio Therapeutics, a subsidiary of the US firm Precigen, is now running a phase I/II clinical trial with an unusual approach to stop the progression of type 1 diabetes. The company uses cheese-producing bacteria to deliver two drugs that stimulate regulatory T cells to instruct the immune system not to attack insulin-producing cells.
“It is potentially a safe oral treatment that will be given for a limited period of time and could lead to patients who develop type 1 diabetes not needing to use insulin, or delay the need for insulin after diagnosis,” said Pieter Rottiers, CEO of Precigen ActoBio.
Automated treatment with an artificial pancreas
For people that have already lost their insulin-producing cells, a shorter-term solution could be the ‘artificial pancreas’ — a fully automated system that can measure glucose levels and inject the right amount of insulin into the bloodstream, just like a healthy pancreas would.
“Type 1 diabetes is very different from your standard disease. Insulin requirements vary greatly from one day to another and there is no way patients can know what they need,” said Roman Hovorka, Professor at the University of Cambridge.
His research group is working on the development of an algorithm that can accurately predict insulin requirements for a specific patient in real-time, which can be used to control insulin delivery via an insulin pump.
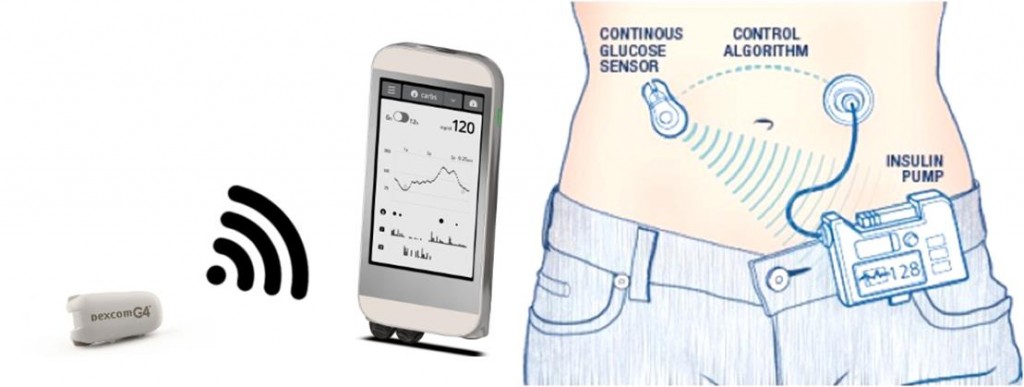
Replacing humans with computers could help patients better control their sugar levels and suffer fewer complications in the long term. However, in order to fully automate insulin therapy, there are several challenges yet to be addressed. First of all, faster forms of insulin are needed to react quickly enough to changes in sugar blood. In addition, current algorithms need to significantly improve to be able to make accurate predictions.
Type 2 diabetes
Stimulating insulin production
“During the past decade, over 40 new pills and injections were approved for diabetes. However, the scary reality is that the majority of patients with type 2 diabetes still have poor glycemic control,” said Kurt Graves, CEO of Intarcia.
One of the biggest hits in type 2 diabetes treatment are glucagon-like peptide (GLP)-1 receptor agonists, which induce insulin production in beta-pancreatic cells while suppressing the secretion of glucagon, a hormone with the opposite effect to insulin.
All big pharma have GLP-1 drugs on the market or their pipelines, including Sanofi, Eli Lilly, Roche, AstraZeneca, and Boehringer Ingelheim. But Novo Nordisk went a step further in 2019 with the approval of the first oral version of the GLP-1 drug semaglutide, which is now on the market.
The French company Poxel is going after a different approach with a drug that simultaneously targets the pancreas, the liver, and the muscles to reduce blood sugar. The drug has proved this effect in a phase III trial in Japan and was consequently approved in summer 2021. Poxel is currently planning a phase III trial for the same drug in Europe and the US, where it will then seek approval as well.
In Sweden, Betagenon and Baltic Bio are working on a first-in-class drug with the potential to simultaneously control sugar levels and reduce blood pressure, a big risk factor in patients with type 2 diabetes who are also obese. The drug is currently in phase II clinical trials.
Tackling the obesity component of type 2 diabetes is also the German MorphoSys, which is running phase II trials with Novartis for an antibody designed to reduce fat, prevent insulin resistance, and control excessive eating.
Targeting the microbiome
In the past decade, scientists have realized the big role that the microbes living inside and on us play in our health. The human microbiome, and especially the gut microbiome, has been linked to multiple chronic diseases, including diabetes.
An unbalanced microbiome composition has been found in patients with diabetes, who tend to have a less diverse gut microbiome as compared to healthy people.
In 2017, researchers from the University of Amsterdam showed that fecal transplants, used to transfer the microbiome of a healthy person to the gut of one with diabetes, can result in a short-term improvement of insulin resistance in obese patients with type 2 diabetes. In 2021, similar results were shown in patients who had recently been diagnosed with type 1 diabetes.
Some companies are developing diabetes treatments targeting the microbiome. The French Valbiotis is currently conducting phase II/III trials for a drug aimed at increasing the microbiome diversity as a treatment for early-stage type 2 diabetes.
Although promising, the microbiome field is very young and its complexity makes it difficult to establish causation after finding correlation. Until more diabetes treatments are tested in the clinic, it will be difficult to determine the real potential of the microbiome in this space.
The needle-free revolution
In order to monitor their blood sugar levels, diabetes patients often need to prick their skin with lancets in an often uncomfortable and painful process. However, many companies are developing non-invasive methods to substitute finger pricking.
Integrity Applications, for example, has developed a device called GlucoTrack that can measure glucose using electromagnetic waves and is already available in Europe.
Similar technologies are popping up, with German firm DiaMonTec using an infrared laser to beam light through the skin of the finger to measure sugar levels and MediWise making use of radio waves. “The device could reduce costs for healthcare, which in the case of diabetes account for €90B a year in Europe,” said MediWise co-founder Panos Kosmas.
Patches are also becoming a popular form of measuring blood glucose without repetitive needle-pricking, such as FreeStyle Libre, an inch-wide patch that can be worn for up to two weeks and that requires a continuous, subcutaneous needle prick to install the device. At the University of Bath, researchers are developing a graphene patch that could provide greater accuracy by measuring sugar levels individually in multiple hair follicles.
Dutch firm NovioSense is going for a tiny device that is placed under the eyelid and would be more affordable than current continuous glucose monitors.
Meanwhile, Senseonics and Roche have developed a glucose monitoring device that is implanted under the skin and lasts for three months, although competition with FreeStyle Libre has been fierce, especially in Europe.
Still, non-invasive options to measure blood sugar often face issues regarding accuracy. The famous glucose-measuring contact lens that Google announced in 2014 was dismissed as “technically infeasible” four years later and further developments will be needed to reach the degree of accuracy of finger-pricking methods.
What’s next in diabetes treatment?
The global diabetes drugs market is expected to reach a massive €68B ($78B) by 2026, and we can expect all sorts of revolutionary technologies to come forward and claim their market share.
Researchers are already speculating about microchips that can diagnose diabetes type 1 before the symptoms appear, nanorobots traveling in the bloodstream while they measure glucose and deliver insulin, or silica particles that can slow down the digestion of food to prevent diabetes and obesity.
Whatever the future brings, it will undoubtedly make a huge difference in the lives of millions of people worldwide.






