During drug development, biologics often fail to transition from the preclinical to the clinical stage, resulting in huge losses in time and money. We explore how choosing the right expression system and optimizing a method known as transient protein expression may offer a potential solution.
Bioproduction is the use of living systems to produce complex therapeutic drugs such as antibodies or enzymes. These living systems, which include bacterial, yeast, and mammalian cells, are referred to as expression hosts, and the resulting drug, a biologic.
During bioproduction, a plasmid — an engineered genetic construct — is delivered to the host cell. The plasmid contains instructions on how to translate the genetic information using the host’s own machinery, and enabling successful protein production.
The entire set-up, consisting of the host cells, plasmid, and conditions used to enable bioproduction, is known as a protein expression system.
The importance of choosing an optimal expression system
The drug discovery and development process is often lengthy, spanning over 10 years on average. It is also expensive, usually requiring billions of dollars in investments.
Therefore, choosing the right expression system is crucial to optimize biologic development and ensure that the heavy investments pay off.
 “The protein’s structure defines its activity,” said Ana Reis, Scientific Content Manager at ProteoGenix, a European antibody production company. For example, if the active centers or binding sites of enzymes and antibodies are not in their proper configurations, the proteins might not interact with their targets or the reaction might not be as effective.
“The protein’s structure defines its activity,” said Ana Reis, Scientific Content Manager at ProteoGenix, a European antibody production company. For example, if the active centers or binding sites of enzymes and antibodies are not in their proper configurations, the proteins might not interact with their targets or the reaction might not be as effective.
This configuration is influenced not just by accurate protein translation, but also by the correct processing of events after translation, referred to as post-translational modifications (PTMs).
“Having proper protein folding and human-like PTMs is crucial to ensure the necessary therapeutic effect. This also ensures that no immunological reactions are caused when the drug is administered to patients,” Reis continued. “When transitioning a biologic from preclinical to clinical stages, having the right expression set-up becomes even more crucial, especially when going from small- to large-scale expression.”
“For example, expression hosts differ in their folding and PTMs processes, and it is inadvisable to change expression systems — such as by switching from one cell type to another — when expanding production. If not, a compound identified as a good candidate drug for further development might not perform as well in later stages due to differences in folding and processing.”
Expression systems: from bacteria to mammalian cells
The first host to be used for protein expression was the bacterial strain Escherichia coli. Bacteria possess genetic machinery that ensures the plasmid is replicated and transferred to their progeny. This enables everlasting and large-scale production of the required proteins.
“However, E. coli produces endotoxins, which have to be removed from the end product for any medical applications,” explained Reis. “Also, they cannot perform complex (human-like) PTMs and do not act as ideal hosts for folding complex proteins, which limits the therapeutic effect of the end product.”
Scientists have also worked with other systems such as yeast, baculovirus-insect, and mammalian cells.
 “In applications such as antibody production, human-like protein processing and superior folding ability is critical. For this, mammalian systems like Chinese hamster ovary cells (CHO) or human embryonic kidney cells (HEK) are preferred” added Isabelle Robert, Lab head and Research Scientist in Molecular and Cellular Biology and Immunology at ProteoGenix.
“In applications such as antibody production, human-like protein processing and superior folding ability is critical. For this, mammalian systems like Chinese hamster ovary cells (CHO) or human embryonic kidney cells (HEK) are preferred” added Isabelle Robert, Lab head and Research Scientist in Molecular and Cellular Biology and Immunology at ProteoGenix.
However, as mammalian cells cannot replicate plasmids when the cells divide, they often lose the plasmids over time. To obtain long-term and stable protein production from mammalian cells, integration mechanisms need to be applied, followed by careful selection of cells that have successfully incorporated the foreign DNA.
Today, several biologics produced in CHO cells have received FDA and EMA regulatory approvals, making this the predominant system for large-scale biopharmaceutical production. To help minimize the risks of transitioning from small- to large-scale production, CHO cells have also become a popular host choice in early development.
The transient expression system: inexpensive and rapid protein production
As bioproduction relies on living systems, it has some associated challenges.
Expression hosts need to be kept alive and thriving in a controlled environment within flexible monitoring systems during the course of bioproduction. To ensure high production yields, the systems need to be free of contamination, and the cells need to retain their capacity to self-replicate and produce proteins in a stable manner.
These measures require time-consuming and costly investments.
During early stages of drug development, large quantities of proteins are not needed. Therefore, a temporary, small-scale production method known as transient protein expression can be used to save time, costs, and resources.
“While traditional, stable expression involves virtually everlasting and large-scale protein production, transient expression produces small quantities for a short amount of time,” elaborated Reis.
“Transient expression does not require stable plasmid integration into mammalian genomes or manual cell selection. It can produce highly complex proteins in a matter of weeks, instead of months, which is ideal for preclinical development.”
The versatile applications of transient systems
Transient expression comes in handy when a wide range of distinct protein molecules need to be produced in limited quantities. It can also enable rapid identification of production issues such as protein aggregation and instability.
A prominent application of transient production is in candidate drug screening, where many promising biologics need to be screened to identify leads for further development.
Similarly, transient expression can help produce the required protein variants in protein engineering, where random or site-directed mutations are induced to improve the properties of a potential drug.
“Transient production is also useful in basic research, particularly in analytical assays to detect proteins or contaminants, or determine their concentrations. It could be used to produce a variety of proteins such as antigens, antibodies, or enzymes for research applications,” continued Reis. “Of late, this method is being used for the in vitro validation of novel in silico-designed biopharmaceutical compounds.”
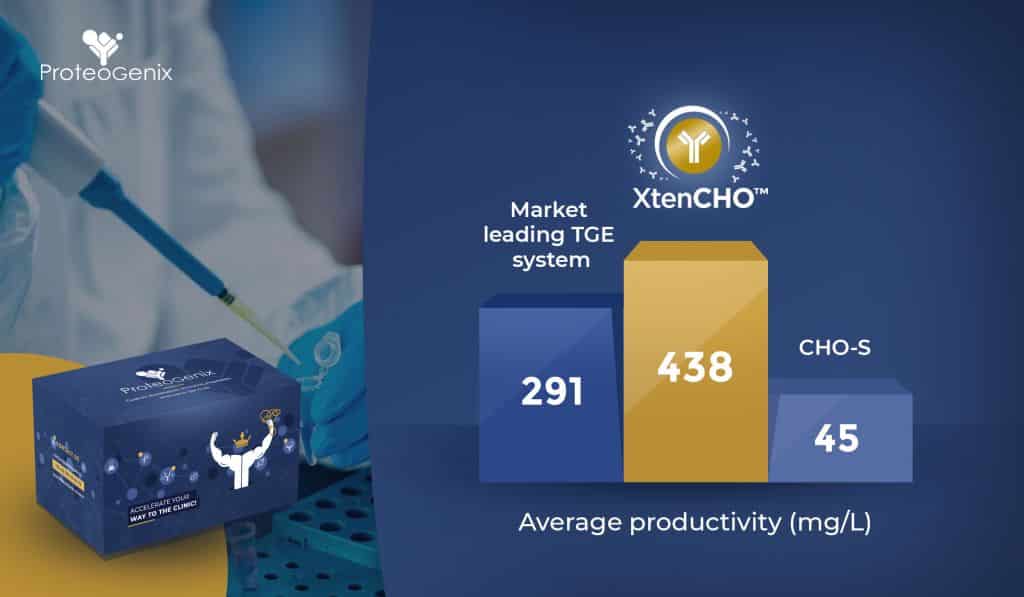
In fact, commercially available expression systems today, such as ProteoGenix’s XtenCHO™, are being designed with these applications in mind. “Leveraging the enhanced bioproduction characteristics of CHO cells and their resistance to viruses, our XtenCHO™ system is easy to integrate into existing biologics discovery workflows,” said Robert.
“The system has low nutrient requirements and high resistance to temperature changes, which saves researcher time in maintaining cells. It has an easy-to-follow protocol with reduced hands-on time, providing a production yield that is 5- to 10-fold higher than that from competitor systems.”
The focus of such systems is also scalability: XtenCHO™ has been used for both small- and medium-scale production in basic research. It boasts an extended transient gene expression period of 14 days, versus the usual 7 to 10 day cell viability seen in other systems.
The future of optimized bioproduction
“CHO cell-based systems are likely to remain the gold-standard expression systems for several years due to their scalability, versatility, human-like protein processing, and a solid track record of successful regulatory approvals,” shared Robert.
“And thanks to multiomics — research that provides insights from biological analyses of the genome, proteome, and more — our knowledge of host cells and their optimization potential is increasing rapidly every day.”
This improved understanding, in combination with editing tools fueled by the CRISPR gene-editing revolution, will help in developing innovative new host cell lines. “As the yield, quality, and targeted activity of the expressed protein gets optimized, it will enable rapid evolution in biologics discovery and bioproduction” concluded Robert.
Learn more about the applications of the XtenCHO™ transient expression system that span across early drug discovery, protein engineering and research.
To collaborate with ProteoGenix and apply rapid, cost-effective solutions to optimize your protein expression process, visit the company website.
This article was originally published in May 2022.
Images via Shutterstock.com and ProteoGenix.